NASOPHARYNGEAL CANCER
DATA UPDATE: Nimotozumab + Chemoradiotherapy (2022)
“Nimotuzumab combined with chemoradiotherapy improves 5-year OS in NPC patients with a safe profile. [1] ”
5-years OS rate vs Safety Profile
5-years OS Rate
Nimotuzumab + Chemoradiotherapy (Arm A) 76.9%
Chemoradiotherapy alone (Arm B) 64.3%
(log-rank=4.125, p=0.042)
vs
Safety Profile
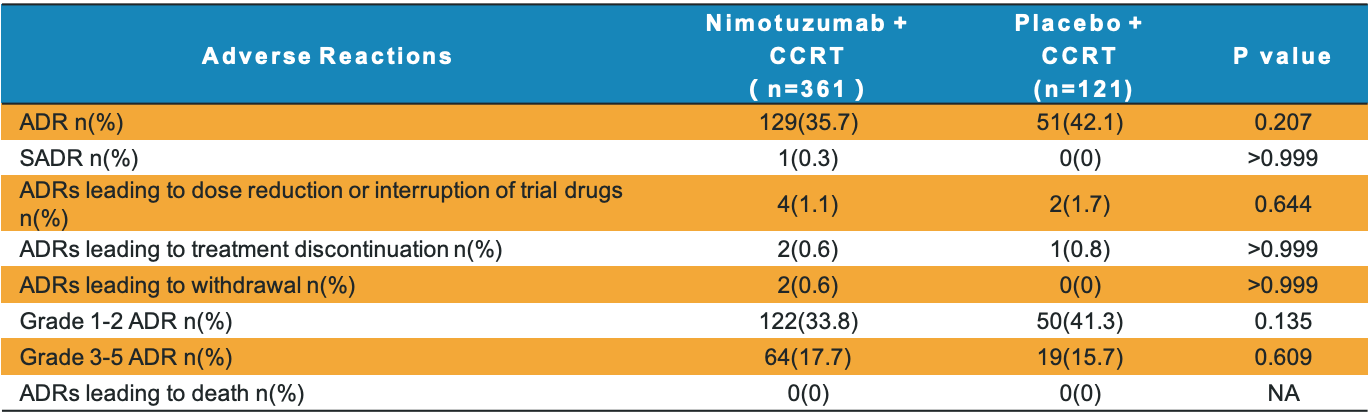
Adverse drug reactions (ADRs) in Arm A was similar to Arm B (35.7% vs. 42.1%, p=0.207)
The grade 3-5 ADRs as well (17.7% vs.15.7%, p=0.609).
S = median overall survival;
Nimotuzumab plus chemoradiotherapy versus placebo plus chemoradiotherapy in patients with locally advanced nasopharyngeal carcinoma (NPC)
Below is an excerpt from a study in China as presented at ASCO (2022), publication of completed study in process: Yan Su et al. (2022)
Study Background & About NPC
- Epidermal growth factor receptor (EGFR) is highly expressed in most NPC, and it is also an essential factor of prognosis for NPC. Nimotuzumab is a humanized anti-EGFR monoclonal antibody.
- Patients with locally advanced nasopharyngeal cancer (NPC) receive advantages of concomitant chemoradiotherapy (CCRT). 2
- In a phase II study of locally advanced NPC patients, the addition of nimotuzumab to chemo-radiation enhanced clinical efficacy. 3
- The current trial is a confirmatory study that aims to evaluate the clinical efficacy, long-term survival, and safety of nimotuzumab combination with chemo-radiotherapy in patients with locally advanced NPC.
Study Design (NCT01074021)
How was Nimotuzumab with chemotherapy studied?
A clinical trial phase III that compared 2 groups with total 483 patients of locally advanced NPC
(Randomized-controlled, Double-blinded, Multicenter Clinical Trial)

Primary endpoint: 5-year OS rate
Secondary endpoints: ORR, DCR, DFS, LRFS, DMR and safety profile
* Objective response rate (ORR), disease control rate (DCR), disease-free survival (DFS), loco-regional recurrence free survival (LRFS), distant metastasis rate (DMR) and safety profile
A sample of 410 patients, the study would have 90% power to detect a difference of 5yr OS rate with nimotuzumab (86.0%) versus placebo (72.3%) at a two-sided alpha level of 0.05 and 20% drop-out. A sample of 483 patients were based on China regulation requirement.
Efficacy

Treatment arms were compared using log-rank tests. The Hazard Risk (HR) with 95% CI was used as estimate of the difference between the arms. Data cutoff, Sept 28,2021.
Reverse Kaplan-Meier was used to estimate follow up duration.
Safety Profile
The incidence of adverse drug reactions (ADRs) in nimotuzumab group was similar to placebo group
ADRs, adverse drug reactions; SADR, serious adverse drug reactions
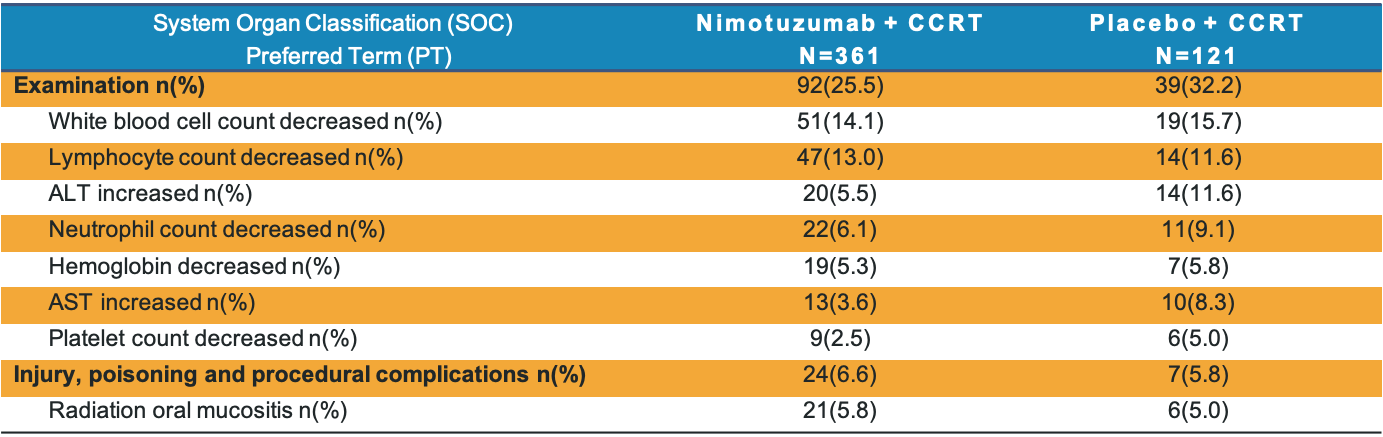
Common Adverse Drug Reactions
- The most common (>5%) adverse drug reactions were various kinds of abnormal examination.
- The incidence of common adverse drug reactions in nimotuzumab group is numerically lower than placebo group.
Conclusion
Nimotuzumab combined with chemo-radiotherapy in the treatment of locally advanced nasopharyngeal carcinoma had a better efficacy:
- 5-year OS rate increased by 12.6% with 36% risk reduction
- Complete response rate increased by 13.3%
Nimotuzumab in combination with chemo-radiotherapy has a good safety profile
Reference
- Sun, Y., Chaosu, H., Qin, L., Gao, L., & Wang, J. (2022). Nimotuzumab plus chemoradiotherapy versus placebo plus chemoradiotherapy in patients with locally advanced nasopharyngeal carcinoma (NPC): A prospective, randomized-controlled, double-blinded, multicenter phase III clinical trial. Journal of Clinical Oncology, 40. https://doi.org/ 10.1200/JCO.2022.40.16_suppl.6001
- Baujat, B., Audry, H., Bourhis, J., Chan, A. T., Onat, H., Chua, D. T., Kwong, D. L., Al-Sarraf, M., Chi, K. H., Hareyama, M., Leung, S. F., Thephamongkhol, K., Pignon, J. P., & MAC-NPC Collaborative Group (2006). Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. International journal of radiation oncology, biology, physics, 64(1), 47–56. https://doi.org/10.1016/j.ijrobp.2005.06.037
- Huang, X. D., Yi, J. L., Gao, L., Xu, G. Z., Jin, J., Yang, W. Z., Lu, T. X., Wu, S. X., Wu, R. R., Hu, W. H., Xie, W. C., Han, F., Gao, Y. H., Gao, J. M., Pan, J. J., Chen, C. B., Lang, J. Y., Li, T., Dong, Y., Fu, Y. B., … Hu, B. Q. (2007). Zhonghua zhong liu za zhi [Chinese journal of oncology], 29(3), 197–201.
Phase III randomised trial (2016)
Locally-Advanced Nasopharyngeal Cancer (NPC)
Nimotuzumab has been studied in 1 clinical trial in NPC indication involving a total of 136 patients in China. There is now 1 ongoing study in China involving more than 482 patients as of August 2018.
For a complete publication and list of clinical trial on nimotuzumab in NPC please contact us here.
Below is an excerpt from a study in China as presented at ASCO (2016), publication of completed study in process: Lin Kong et al. Radiation plus concurrent nimotuzumab versus cisplatin for locally advanced nasopharyngeal cancer. Interim Analysis, presented in ASCO Annual Meeting 2016.
Disease state
- Nasopharyngeal carcinoma (NPC) is the most common primary malignant tumour of the nasopharynx.
- The incidence of NPC shows considerable variation in different regions of the world. It is a relatively uncommon cancer in the West NPC has an intermediate incidence of 5 to 9 cases per 100,000 population per year in Northern China, the Mediterranean basin (Southern Italy, Greece and Turkey), North Africa and Southeast Asia (Thailand, Vietnam, Indonesia, Malaysia and Singapore). It is particularly common in Southern China where the incidence rates may range from 10 to 150 per 100,000 population.
- NPC affects a relatively younger age group of patients. Survival at 5 years was 72% for the youngest age group (15–45 years) and 36% in the oldest group of patients (65–74 years).
- About 80% to 90% overexpression of EGFR was reported in NPC. Increased expression of EGFR in advanced stage NPC patients has been reported and is associated with poor survival.
- The standard line of therapy used in locally advanced unresectable NPC is concurrent chemoradiotherapy with or without adjuvant chemotherapy.
Study Design
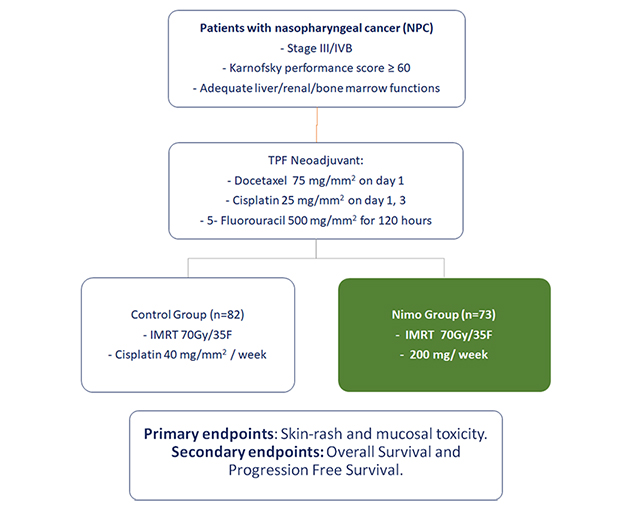
The percentages of patients showing skin rash and mucosities were lower in nimotuzumab group compared to the control group.
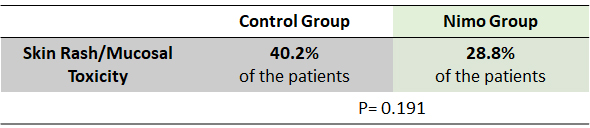
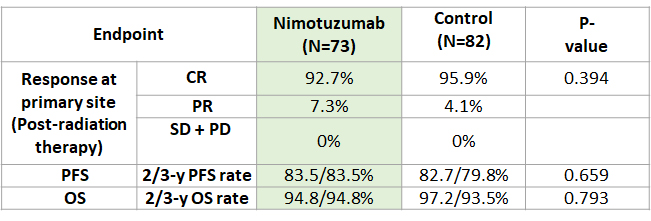
Nimotuzumab produces similar PFS and OS as compared to control group with concurrent RT for LA-NPC on 2 and 3-year follow up
Patients treated with Nimotuzumab following the TFP induction chemotherapy showed significantly less GI and hematologic toxicities compared to the patients in control group.