Head & Neck Cancer
2-years Progression-free Survival of Nimotuzumab + Chemoradiation (NCRT)
61.8% with Nimotuzumab + Chemoradiation
(95% CI, 55.2-67.7)
(HR=0.69; 95% CI, 0.53-0.89; P = 0.004)
vs
50.1% with Chemoradiation
(95% CI, 43.7-56.2)
PFS = progression-free survival; HR = hazard ratio; CI = confidence interval
Nimotuzumab for Locally Advanced Head and Neck Squamous Cell Carcinoma (LAHNSCC)
A total of more than 46 clinical trials have been completed with Nimotuzumab for various cancer indications worldwide, with more than 4382 patients enrolled in the trials. Nimotuzumab has been studied in ten clinical trials at HNSCC, conducted in Canada, Cuba and India. In addition, there are ongoing studies in Singapore and Cuba involving nearly 500 patients until August 2021.
Below is a summary of one of our phase III clinical trials in LAHNSCC:
Patil et al. A randomized phase 3 trial comparing nimotuzumab plus cisplatin chemoradiotherapy versus cisplatin chemoradiotherapy alone in locally advanced head and neck cancer. Cancer. 2019 Sep 15;125(18):3184-3197.
Study Background
Head and neck cancer is the sixth most common cancer worldwide, with 630,000 new diagnoses and 350,000 annual deaths.
Read More
Head and neck cancer represents the sixth most common cancer worldwide with approximately 630,000 new patients diagnosed and 350,000 deaths annually. More than 90% of head and neck cancers are squamous cell carcinomas and 60% of them are diagnosed at a localized or locally advanced stage.
LAHNSCC is treated using a multimodality approach. Radical chemoradiation is the nonsurgical approach of choice and is associated with improved survival, but the outcomes are modest with a 3-year overall survival (OS) rate of approximately 50%. Thus, efforts to improve the outcomes using EGFR-targeted antibodies have been made.
Among EGFR-targeting antibodies, Nimotuzumab is a humanized immunoglobulin G1 isotype monoclonal antibody directed against the extracellular domain of EGFR. A randomized phase 2b and phase 3 study at LAHNSCC found that nimotuzumab + cisplatin chemoradiation achieved a higher response rate and progression-free survival (PFS) than cisplatin chemoradiation only. [1,2]
Study Design
A clinical trial phase III that compared 2 groups with total 536 adult patients of locally advanced head and neck cancer (randomized, open-label, two arms).
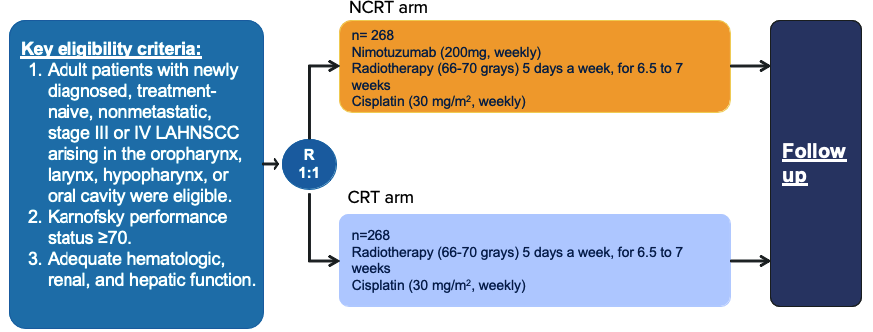
Primary endpoint: PFS
Secondary endpoints: DFS, LRC and OS
* PFS, progression-free survival; DFS, disease-free survival; LRC, locoregional control; OS, overall survival;
Efficacy
The Addition of Nimotuzumab to Chemoradiation Improves PFS, LRC and DFS
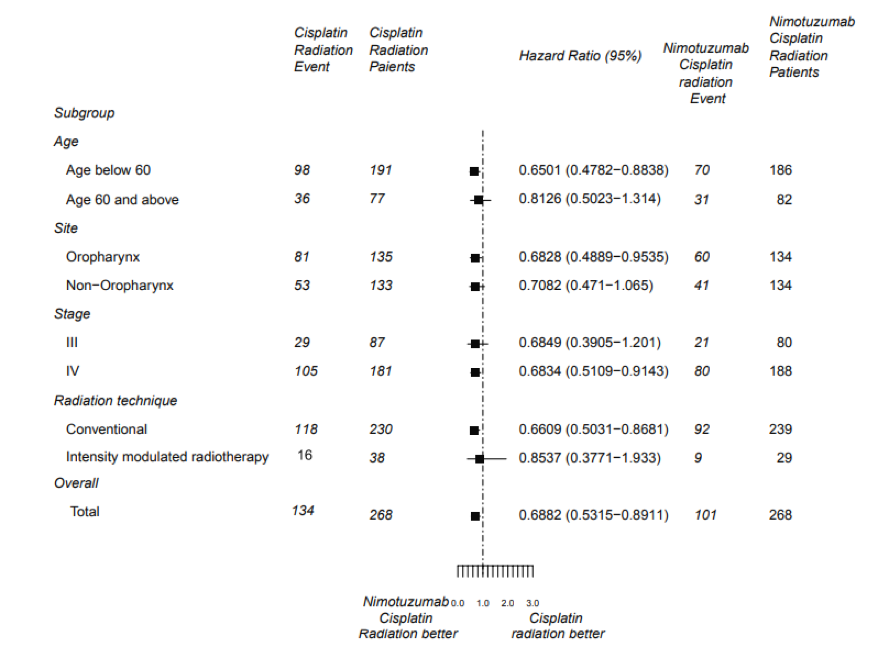
There were 134 and 101 patients in the Chemoradiation (CRT) and Nimotuzumab + Chemoradiation arm (NCRT) arm, respectively, who had disease progression. The PFS was significantly longer in NCRT arm (HR=0.69; 95% CI, 0.53-0.89; P = 0.004).

Furthermore, the addition of nimotuzumab led to a consistent benefit across all subgroups.
Nimotuzumab also improved the secondary endpoints:
A. LRC: NCRT arm had a 33% reduction (HR=0.67; 95% CI, 0.50-0.89; P =.006) in the risk of locoregional failure.
B. DFS: NCRT arm had a smaller hazard of disease recurrence by 29% (HR=0.71; 95% CI, 0.55-0.92; P =.008).
C. OS: NCRT arm had a lower mortality number (HR=0.84; 95% CI, 0.65-1.08; P =.163).
Safety Profile
All adverse events occurred with similar frequency in both arms except mucositis and thrombocytopenia
Read More: Nimotuzumab is safe and well-tolerated
The safety of Nimotuzumab in cancer patients is very well-known compared to other agents, (including other EGFR-mAbs) and it can be seen in many completed clinical trials. In this trial, the same conclusion was observed that almost all adverse events occurred with similar frequency in the 2 arms. With the exception of grade ≥3 mucositis, however it did not hamper the treatment delivery. This can be explained by the gastro-intestinal tract’s higher density of EGFR receptor than skin and other normal organs.

Conclusion
The PFS, LRC, and DFS were improved in addition of Nimotuzumab to concurrent weekly cisplatin and chemoradiotherapy.
Reference
- Patil VM, Noronha V, Joshi A, et al. A randomized phase 3 trial comparing nimotuzumab plus cisplatin chemoradiotherapy versus cisplatin chemoradiotherapy alone in locally advanced head and neck cancer. Cancer. 2019;125(18):3184-3197. doi:10.1002/cncr.32179
- Reddy BK, Lokesh V, Vidyasagar MS, et al. Nimotuzumab provides survival benefit to patients with inoperable advanced squamous cell carcinoma of the head and neck: a randomized, open-label, phase IIb, 5-year study in Indian patients. Oral Oncol. 2014;50(5):498-505. doi:10.1016/j.oraloncology.2013.11.008
